How Many Valence Electrons Does Roentgenium Have
Vanadium has 2 valence electrons because if you look at the electron configuration the first shell has 2 the second 8 the third 11 and the last shell where the valence electrons are has 2. Each electron is influenced by the electric fields produced by the positive nuclear charge and the other Z 1 negative electrons in the atom.

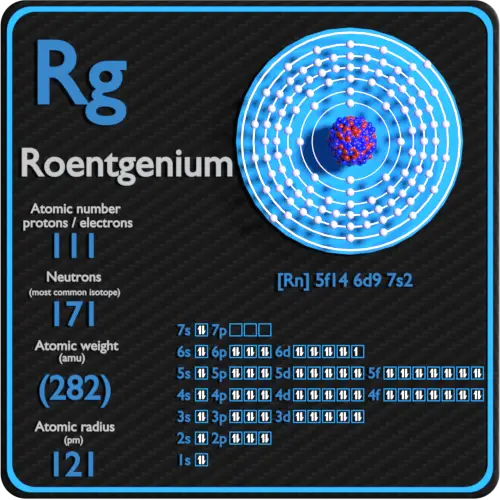
Roentgenium Electron Configuration Symbol Atomic Number Atomic Mass Oxidation States Standard State Group Block Year Discovered
The chemical symbol for Roentgenium is Rg.
. Roentgenium Atomic Structure Stock Image C045 6343 Science Photo Library This leaves only three electrons in the third shell 3s23p1. What is the valency of lead. Members of a group typically have similar properties and electron configurations in their outer shell.
Bromine as a configuration of Ar4s 2 3d 10 4p 5. The atomic number of each element increases by one reading from left to right. A horizontal row in the periodic table.
Crystal structure of Roentgenium. So it has 3 electron shells and that will be its valence shell. Electron configuration of Roentgenium is Rn 5f14 6d9 7s2.
Potassium has an atomic number of 19 which means one neutral atom of this element has 19 electrons. Roentgenium is a chemical element with atomic number 111 which means there are 111 protons and 111 electrons in the atomic structure. Valence electrons are the electrons present in the outermost shell of an atom.
Rn 5f 14 6d 9 7s 2. Solid presumed Melting point. 272 274 and 278-282.
Does Ar have 8 valence electrons. For main group elements ie s-block and p-block elements the valence electrons are the electrons present in the outermost orbit. Therefore the Lewis electron dot formula for magnesium is.
Number of neutrons Atomic mass of a given isotope of Rg - 111. Thallium electron configuration is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 4f 14 5s 2 5p 6 5d 10 6s 2 6p 2. How many protons neutrons and electrons are in a roentgenium atom.
A vertical column in the periodic table. Neutrons in Roentgenium. There are 2 valence electrons as indicated by.
Ok but how many valence electrons does an atom of Ruthenium have. Valence Electrons Graph - Valence Electrons of all the elements in graph. Valence electrons in Roentgenium Rg 11.
In our example since carbon is in group 14 we can say that one atom of carbon has four valence electrons. Find an element from Groups 3 to 12. Valence electrons in Nihonium Nh 3.
In the case of Ruthenium the valence electrons is 012345678. You can easily determine the number of valence electrons an atom can have by looking at its Group in the periodic table. This adds up to 23 total electrons the atomic number of vanadium is 23.
As noted above the elements in groups 3 to 12 are called transition metals and behave differently than the rest of the elements when it comes to valence electrons. Electrons and Electron Configuration. Valence electrons in Copernicium Cn 12.
Atomic mass of Roentgenium most stable isotope 282 u. The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus. Gold also forms a somewhat stable 1 state due to relativistic effects and it has been suggested roentgenium may do so as well.
2 8 18 32 32 17 2. Electrons arrangement in Roentgenium or Bohr model of Roentgenium. The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus.
How many valence electrons does Bromine have. Therefore the number of electrons in neutral atom of Roentgenium is 111. A valence electron is an outer shell electron and may participate in the formation of a chemical bond.
Ruthenium Overview Ruthenium Valence Electrons 012345678. The valency of lead is 2 and 4. The electron configuration for chlorine Cl could be written as.
113 Nh - Nihonium. Thus in its valence shell it has 2 4s electrons and 5 4pelectrons. Electronic configuration of Roentgenium Rn 5f 14 6d 9 7s 2.
Therefore the maximum electron holding capacity in the first shell is two the second shell is eight and the 3rd shell can have a maximum of eighteen electrons. Electron Configuration and Oxidation States of Roentgenium. Density 20 o C.
Atoms in Groups 13 and 18 have 3 and 8 valence electrons. 111 Rg - Roentgenium. Is 18 units too much for the average college student.
Neutrons in most abundant isotope. Characteristics of Valence Electron. Elements like barium located toward the bottom of a group have a lower attraction for their valence electrons because they have a.
Roentgenium is a transition metal. Bromine has 7 valence electrons. The maximum electron holding capacity in N orbit is 2n 2 2 4 2 32 electrons.
It is said to occupy orbitals in an atom. How many valence electrons does magnesium have. 112 Cn - Copernicium.
The number of valence electrons of an atom can be obtained from the periodic table because it is equal to the group number of the atom. Roentgenium has 111. Atoms are most stable if they have a filled valence shell.
How many electrons does lead have. Argon is a noble gas with an electron configuration of Ne3s23p6. Each electron is influenced by the electric fields produced by the positive nuclear charge and the other Z 1 negative electrons in the atom.
The core electrons would be while the valence electrons would be in. Nevertheless the electron affinity of roentgenium is expected to be around 16 eV 37 kcalmol significantly lower than golds value of 23 eV 53 kcalmol so roentgenides may not be stable or even possible. 281 no stable isotopes.
For example atoms in Groups 1 and 2 have 1 and 2 valence electrons respectively. The number in the last group is the amount of valence electrons. How many electrons does roentgenium have.
Therefore the number of electrons in neutral atom of Roentgenium is 111. Look at the electron configuration for chlorine. Therefore the valence electrons of vanadium are five.
Valence electrons in Flerovium Fl 4. Electrons are involved in the chemical bonding and reactions of the atom. I am vollenterring at an.
Possible oxidation states are. N4 for N orbit. We have shown the Valence Electrons of the elements for which reliable data is available.
How do you write the electron configuration for lead. Roentgenium has 111 protons an 111 electrons. Now lets check the facts about Ruthenium.
In the third shell it holds a total of 268 electrons so it has 8 valence electrons.

Roentgenium Protons Neutrons Electrons Electron Configuration
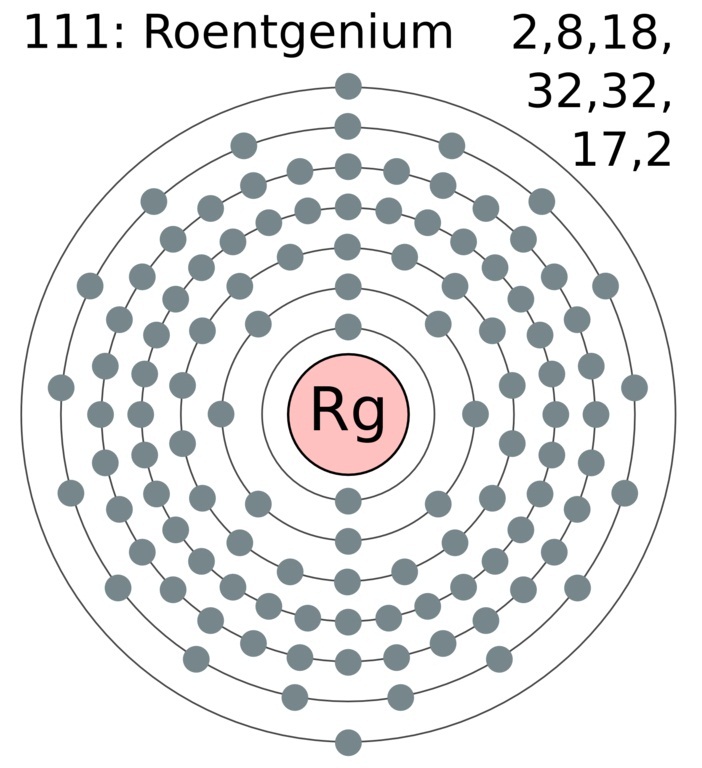
Roentgenium Valence Electrons Roentgenium Valency Rg Dot Diagram

Roentgenium Atomic Structure Stock Image C024 8611 Science Photo Library

Rutherfordium Art Print By Carlos Clarivan Electron Configuration Atomic Structure Gold Art Print
Comments
Post a Comment